

← Home • Knowledge • Demineralised Water ↓ Definitions • Ion Exchange • Membrane Processes• Economic Considerations • Power Plants • Industrial Shell Boilers
Demineralised water (short: demin water) is water where the electrolyte concentration has been significantly reduced by technical processes. However, the detailed definition of demineralised water varies depending upon context and application. Usually, such definitions include limit values for the →electrical conductivity, serving as non-specific indicator for the electrolyte concentration.
| code | EN 12952-12 | EN 12953-10 | VGB-M 407 | VGB-S-010-T-00 | |
| electrical conductivity | < 0.2 μS/cm | ≤ 0.08 μS/cm | |||
| sodium | – | ≤ 5 μg/L Na | |||
| silicic acid | < 20 μg/L SiO2 | ≤ 10 μg/L SiO2 | |||
| dissolved organic carbon (DOC) | – | ≤ 0.2 mg/L C | – | ||
Table 1: Definitions of demineralised water according to different European codes.
In European codes for steam boiler operation, demineralised water is defined as having a quality close to chemically pure water, with the goal of defining make-up water quality requirements for direct contact desuperheating or steam turbine operation. In a broader sense, the term demineralised water is usually understood to also include reverse osmosis permeate with an electrical conductivity of for example ≤ 30 μS/cm, deionised water with an electrical conductivity of usually 0.2 ... 20 μS/cm, but also ultra-pure water with an electrical conductivity of 0.055 μS/cm.
In some cases, the terms polished water or purified water are used synonymous with demineralised water, however both may also simply refer to "clean" water, e.g. filtrated water with a low turbidity and a low concentration of suspended solids. Desalted sea water, with an electrical conductivity of ≤ 1000 μS/cm, is usally not considered to be demineralised water, even though the generally same treatment processes have been applied.
Unless a specific definition is either given or obvious from context, the term demineralised water thus leaves some margin for interpretation.
| denomination | demineralised water (general) | ultra-pure water (pharmaceutical) | deionised water | demineralised water (European codes) | ultra-pure water (semi-conductor) | chemically pure water |
| treatment process | ion exchange, membrane separation, distilliation | ion exchange, membrane separation, distilliation | ion exchange | ion exchange, membrane separation | – | |
| electrical conductivity | 0.055 ... 50 μS/cm | ≤ 1.1 μS/cm | 0.2 ... 20 μS/cm | < 0.2 μS/cm | 0,055 μS/cm ≈ 18 MΩ ⋅ cm | |
Table 2: Different denominations for demineralised water based upon the electrical conductivity and treatment processes.
Note: The content of this section is different from the content of the corresponding →German-language page, as the term demineralised water can be translated into German language in two different ways, neither of which conveys the exactly same meaning.
By ion exchange demineralisation, cations dissolved in water are exchanged for hydrogen ions, while dissolved anions are exchanged for hydroxide ions; both together react to water. Ion exchangers are regenerated with acids and caustics. Accordingly, the operation of ion exchange plants requires equipment for storing and handling of chemicals, as well as equipment for effluent neutralisation. With a given plant design, both the chemical consumption and the effluent discharge are directly proportional to the total dissolved solids (TDS) concentration of the raw water.
The first step of ion exchange demineralisation is deionisation by →cation and anion exchangers arranged in series. With modern plants operating in counter-flow regeneration, the electrical conductivity of the deionised water is approximately 0.2 ... 2 μS/cm.
For further polishing of the deionised water, a →mixed bed exchanger is used downsteam of the anion exchanger. Water polished by a mixed bed exchanger will usually have an electrical conductivity of ≤ 0.08 μS/cm and a silicic acid concentration of ≤ 10 μg/L, thus conforming to any ↑definitions by European standards for steam turbine operation.
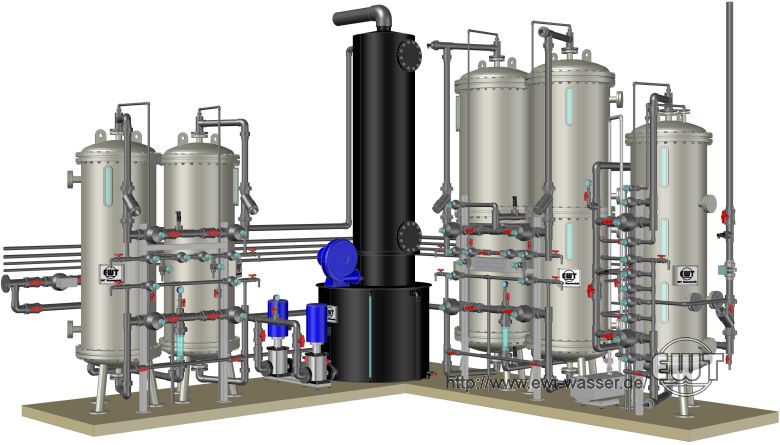
Ion exchange demineralisation plant: Cation exchanger, degasser, anion exchanger, mixed bed polisher.
Ion exchange processes for demineralisation of water on a technical scale were first developed in the 1930s to 1940s, and have been used widely starting in the 1950s. Modern counter flow processes for regeneration have been introduced in the early 1970s, and were further optimised in the 1990s with the development of monospheric ion exchange resins.
Membrane separation processes for demineralisation of water are based upon rejection of ions dissolved in water by a semi-permeable membrane. The ions thus rejected remain within a concentrated aqueous solution, which is being discharged continuously. The waste water discharge and accordingly also the raw water consumption of membrane processes are thus both comparably high, however do not change significantly with the raw water quality for a given plant design.
Membrane processes do not directly consume chemicals for operation. However, some kind of feed treatment involving regeneration with chemicals, e.g. →ion exchange softening, or some kind of chemical feed conditioning are required almost without exception, in addition to periodic chemical cleaning of the membrane elements. This means that membrane separation processes for an overall "chemical-free" demineralisation of water do basically not exist. Regardless, the overall chemical consumption is usually significantly lower compared to ion exchange demineralisation, meaning that extensive equipment for storing and handling or chemicals or for effluent neutralisation is usually not required.
The first step of demineralisation by membrane separation is achieved with a →reverse osmosis plant. The water thus demineralised is called permeate. The electrical conductivity of the permeate is about 5 ... 30 μS/cm in case of a single pass high pressure reverse osmosis plant, about 30 ... 100 μS/cm in case of a low pressure reverse osmosis plant, and about 0.2 ... 2 μS/cm in case of a two-pass plant.
For polishing of the reverse osmosis permeate, an →EDI plant is used. The water thus polished will under favourable conditions have an electrical conductivity of ≤ 0.08 μS/cm and a silicic acid concentration of ≤ 10 μg/L, thus conforming to any ↑definitions by European standards for steam turbine operation. The effectiveness of EDI plants varies significantly with the feed quality, requiring feed with both a low total dissolved solids (TDS) concentration and a low carbon dioxide concentration. The upstream treatment steps need to be designed accordingly, for example with a →membrane degasser arranged upstream of the EDI plant, or a dosing →dosing system for sodium hydroxide combined with a two pass reverse osmosis plant. In order to ensure a reliable demineralised water quality even in case of feed quality fluctuations, it is common practice to arrange a mixed bed polisher downstream of the EDI plant.
As an alternative to the above, the polishing of the reverse osmosis permeate can also occur with a mixed bed exchanger only, or with an arrangement of cation exchanger → anion exchanger → mixed bed.
Reverse osmosis as a process for demineralisation of water on a technical scale was developed in the 1960s, has been used increasingly starting in the 1980s, and gained acceptance as a standard process with the development of spiral-wound membrane modules in the middle of the 1980s. Electrodeionisation as a process for polishing of demineralised water on a larger technical scale was developed in the early 1980s, has been used in slowly increasing amounts since the late 1980s, and has been established as an alternative treatment process since the early 2000s.
In order to compare the economic viability of different demineralisation plant designs, both the investment costs and the operating costs need to be determined based upon the raw water source, the raw water quality, raw water costs, waste water discharge costs, electricity costs, and costs for any consumables
The investment costs for ion exchange plants and membrane seperation plants are usually close to each other with standard designs; more detailed assessments require the consideration of each individual case. Considering the required equipment for storing and handling of chemicals or for effluent neutralisation, the overall investment costs for ion exchange plants are often a little bit higher. However, this equipment may be required anyways for an ion exchange condensate polishing plant, thus allowing for a consideration of only the split costs. Due to intermittent operation, ion exchange plants are usually designed with 100% redundancy, which is not required for membrane plants. Accordingly, a membrane plant with a lower redundancy may in theory still have a higher availability than an ion exchange plant. On the other hand, a membrane plant with identical redundancy will be about 50% to 100% more expensive compared to an ion exchange plant. Thus, a direct comparison of redundancy and availability versus investment costs is not easily possible.
The personnel costs for operation and maintenance of ion exchange and membrane separation plants are approximately equal. However, the material costs for maintenance – meaning the costs for ion exchange resins or membrane elements, divided by the respective service life – may be significantly higher for membrane separation plants, up to approximately 250% ... 500% of the costs for ion exchange plants. This is mainly due to the still relatively high costs for EDI modules, which usually need to be replaced completely in case of failure.
There are significant differences between ion exchange and membrane separation processes regarding the consumption of consumables. For demineralisation of fresh water, ion exchangers consume significantly less water compared to membrane separation processe, however may in turn consume significantly more chemicals. With a given plant design, both chemical consumption and waste water discharge of ion exchange plants are approximately linearly dependent on the total suspended solids (TDS) concentration of the raw water, while neither is significantly related for membrane separation plants. In case of low TDS water (electrical conductivity < 500 μS/cm), ion exchange is often more economical, while membrane separation processes or combinations of membrane separation with ion exchange are often more economical in case of high TDS water (electrical conductivity ≥ 1000 μS/cm).
Depending upon the source and quality of the raw water, possibly extensive pre-treatment steps may be required upstream of both ion exchange or membrane separation plants. For example, →multi-media filter plants, →ultrafiltration plants, →activated carbon filter plants, or →ion exchange scavenger plants may be used, in order to remove for example suspended solids, iron, manganese, organic carbon compounds (TOC), or oxidising agents like chlorine. In such cases, both the investment costs and the oporating costs will be affected accordingly.
| process | ion exchange | membrane separation |
| typical process sequence | cation exchanger → (CO2 degasser) → anion exchanger → mixed bed polisher | (softener) → reverse osmosis → (softener) → (CO2 degasser) → EDI → (mixed bed polisher) |
| raw water | ≤ 1.01 ... 1.1 m³/m³ | approx. 1.18 ... 1.40 m³/m³ |
| waste water discharge | approx. 3 ... 12 L/m³ for each 100 μS/cm electrical conductivity | approx. 180 ... 400 L/m³ |
| electrical energy | approx. 0.1 ... 0.3 kWh/m³ | approx. 0.5 ... 2.5 kWh/m³ |
| other consumables | approx. 40 ... 80 g HCl/m³ and 20 ... 150 g NaOH/m³ for each 100 μS/cm electrical conductivity | With an upstream softener plant approx. 200 ... 400 g NaCl/m³ for each 1 mmol/L water hardness, with a downstream softener instead ≤ 2 g NaCl/m³ and approx. 2.5 ...7 g antiscalant/m³. |
| equipment service life |
Replacement of the cation exchange resins every ≥ 10 years. Replacement of the anion exchange resins every 5 ... 10 years. |
Replacement of the cation exchange resins every > 10 years. Replacement of the RO membrane elements every 3 ... 10 years. Replacement of the EDI modules every ≥ 5 ... 10 years. |
| maintenance | External cleaning of the ion exchange resins, usually every ≥ 36 months, in case of unfavourable raw water quality every 6 ... 12 months. |
Cleaning of the RO membrane elements, usually every ≥ 36 months, in case of unfavourable raw water quality every 6 ... 12 months. Cleaning of the EDI modules, usually every ≥ 36 months. |
Table 3: Comparison of specific consumable consumption and other parameters relevant for determining operating costs.
In boiler feed water treatment, demineralised water with an electrical conductivity of < 0.2 μS/cm is used as →make-up water for steam turbine plants. The field of applications ranges from small to medium-sized bio mass power plants, waste-to-energy plants, or industrial power plants to large power plants for supra-regional electricity supply. For these applications, both ↑ion exchange or ↑membrane separation processes are used for demineralisation.
For →condensate demineralisation however, only ion exchange processes are feasible from both a technical and economic point of view. This is due to the much higher temperature resistance of ion exchange resins compare to membrane modules, but also due to usually rather low total dissolved solids (TDS) concentration of condensate, resulting in very low operating costs for ion exchange processes.
In case of industrial shell boiler plants, demineralisation serves for producing make-up water with an electrical conductivity of ≤ 30 μS/cm. This allows for an economic operation of the steam boiler plant with boiler water blow-down rates of ≤ 2%, compared to maybe 5 ... 10% when using softened water instead of demineralised water. Almost always, a →reverse osmosis plant is used for that purpose. Depending upon the particular operating conditions and the raw water quality, certain ion exchange processes may in some cases turn out to be more economical in theory, for example →dealkalisation or →ion exchange demineralisation. However, the then required storing and handling of acids and caustics is more often than not considered to be a significant disadvantage for such applications.
2018-05-05 • water treatment made in Germany • Company Information • Privacy