

← Home • Products • Dealkalisation ↓ Summary • Design Example • Applications • Technical Data • Downloads
Dealkalisation (also: dealkalization) is an ion exchange process for partial demineralisation of water by exchanging certain calcium and magnesium ions for hydrogen ions.
For dealkalisation, water flows through a vessel filled with weak acid cation exchange resin in hydrogen form. This results in an amount of calcium and magnesium ions which is chemically equivalent to the alkalinity of the that water to be exchanged for hydrogen ions. The hydrogen ions then react with hydrocarbonate to carbonic acid, and further to water and carbon dioxide. For removal of that carbon dioxide, a CO2-degasser is often used downstream of a dealkalisation plant.
Dealkalisation is a discontinuous process. The ion exchange resins eventually deplete, and then need to be regenerated with either a hydrochloric acid or a sulfuric acid solution. A certain amount of acidic effluent is produced with each regeneration. Compared to other ion exchange processes, dealkalisation requires significantly less regeneration chemicals; the chemical demand is in fact almost stoichiometric.
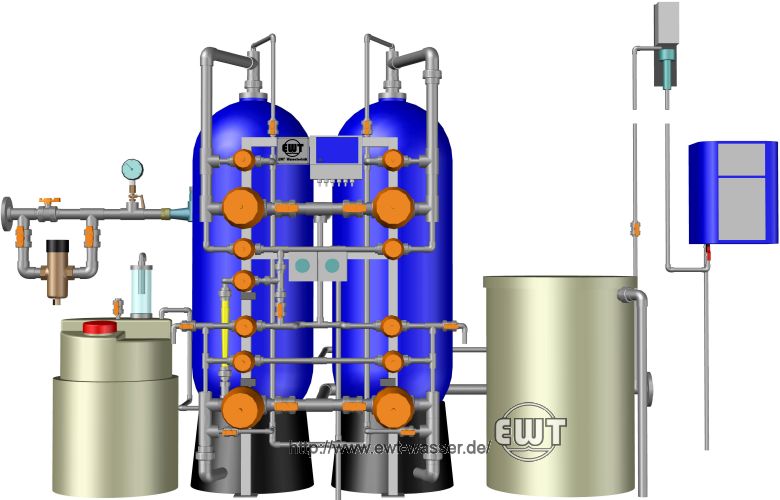
Dealkalisation plant, two lines, with day tank, continuous flow neutralisation plant, and downstream hardness monitor with sampling degasser.
| dealkalised water volume flow | available from approx. 1.5 up to 350 m³/h for each line | ||
| amount of lines | usually 2x100% or 1x100% | ||
| dealkalised water quality | pH value | 4.3 ... 6.5 | |
| electrical conductivity | reduction by up to 50 μS/cm for each 1 mmol/L alkalinity | ||
| water hardness | reduction by up to 0.5 mmol/L for each 1 mmol/L alkalinity | ||
| m-alkalinity | ≤ 0.2 mmol/L | ||
| differential pressure at nominal flow | approx. 1.0 ... 1.5 bar | ||
| operating temperature | usually 10 ... 40 °C | ||
| regeneration agent |
usually hydrochloric acid (HCl) as an alternative sulfuric acid (H2SO4) |
||
| regeneration intervals | usually 6 ... 24 h | ||
| regeneration duration | approx. 60 ... 120 minutes | ||
| chemical consumption | approx. 38 g HCl or 103 g H2SO4 for each 1 mol Ca2+ (¹) | ||
| waste water discharge | approx. 2 ... 15 L for each 1 mol Ca2+ (¹) | ||
| recommended raw water quality | total suspended solids (TSS) | < 1 ... 5 mg/L | |
| iron | < 0.5 mg/L Fe | ||
| manganese | < 0.1 mg/L Mn | ||
| free chlorine | < 0.1 ... 0.3 mg/L Cl2 | ||
| oil, grease | < 0.1 mg/L | ||
| ion exchange medium | cation exchange resin, weak acid, polyacrylic | ||
| total service life of the ion exchange resins | usually ≫ 10 years | ||
| material options | pressure vessel |
• glass-reinforced plastic (GRP) • carbon steel (e.g. S235JR, P265GH) • stainless steel (e.g. 1.4404, 1.4571) |
|
| chemical day tank | • polyethylene (PE) | ||
| pipelines |
• polyvinyl chloride (PVC) • polypropylene(PP) • Polyvinylidene fluoride (PVDF) • stainless steel (e.g. 1.4404, 1.4571) |
||
| valves |
• polyvinyl chloride (PVC) • polypropylene(PP) • Polyvinylidene fluoride (PVDF) • stainless steel (e.g. 1.4408) |
||
| gaskets |
• etyhlene propylene diene monomer rubber (EPDM) • fluoroelastomer (FKM) • polytetrafluoroethylene (PTFE) |
||
| control options | regeneration, line change-over |
• fully automated, via micro-processor control unit • fully automated, via PLC • manual operation (usually not recommended) |
|
| process monitoring |
• volume counter (standard) • differential pressure (option) • pH value (option) • electrical conductivity (option) • residual water hardness (option) • residual alkalinity (option) |
||
| ¹ Chemical equivalent relations: 1 mol Ca2+ ∼ 2 mol HCO3- ∼ 0.1 kg CaCO3, see →unit conversions. Example: In case of water water with an m-alkalinity of 2.5 mmol/L, corresponding to 2.5 mmol/L HCO3- ∼ 1.25 mmol/L Ca2+, a water hardness of ≥ 1.25 mmol/L, and a volume flow of 10 m³/h, the mean chemical consumption is 1.25 mol/m³ ⋅ 10 m³/h ⋅ 38 g HCl = 475 g/h HCl, and the mean waste water discharge is 1.25 mol/m³ ⋅ 10 m³/h ⋅ 2 ... 15 L = 25 ... 188 L/h. | |||
–
2018-05-05 • water treatment made in Germany • Company Information • Privacy