

← Home • Products • Softening ↓ Summary • Design Example • Process Description • Applications • Technical Data • Downloads
Softening is an ion exchange process for removal of calcium and magnesium ions from water. The concentration of these ions in water is also called →water hardness.
A softener is a vessel filled with a resin bed, consisting of strong acid cation exchange resin in sodium form. While water flows through the resin bed, calcium and magnesium ions dissolved in that water are exchanged for sodium ions. Accordingly, softening is not a demineralisation process. Instead, the →equivalent concentration of the total dissolved solids remains constant before and after softening.
With increasing service life, the ion exchange resin depletes, and then needs to be regenerated with sodium chloride brine.
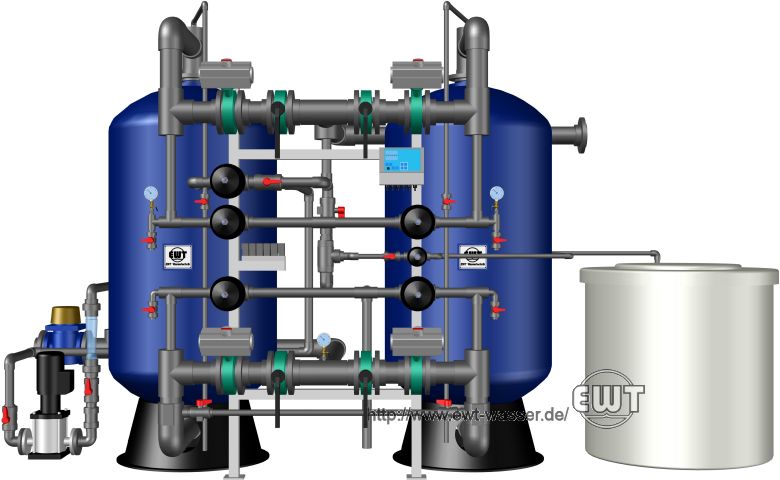
Softening plant, with individually actuated valves, two lines, fully automated counter-flow regeneration, including circulating pump.
Softening is an ion exchange process for removal of the alkaline earth ions calcium (Ca2+) and magnesium (Mg2+) from water by exchanging them for a chemically equivalent amount of sodium ions (Na+). For this purpose, the water flows through a resin bed consisting of strong acid ion exchange resin in sodium form. Ion exchange reactions occur, which can be illustrated as follows:
Ca2+(aq) + 2Cl-(aq) + 2Na+— [resin] ↔ 2Na+(aq) + 2Cl-(aq) + Ca2+— [resin]
Ca2+(aq) + HCO32-(aq) + 2Na+— [resin] ↔ 2Na+(aq) + HCO32-(aq) + Ca2+— [resin]
Ca2+(aq) + SO42-(aq) + 2Na+— [resin] ↔ 2Na+(aq) + SO42-(aq) + Ca2+— [resin]
Mg2+(aq) + 2Cl-(aq) + 2Na+— [resin] ↔ 2Na+(aq) + 2Cl-(aq) + Mg2+— [resin]
Mg2+(aq) + HCO32-(aq) + 2Na+— [resin] ↔ 2Na+(aq) + HCO32-(aq) + Mg2+— [resin]
Mg2+(aq) + SO42-(aq) + 2Na+— [resin] ↔ 2Na+(aq) + SO42-(aq) + Mg2+— [resin]
The above ion exchange rections are reversible, meaning they may occur in both directions. However, there exists a significant concentration gradient, as the raw water contains significanctly less sodium ions and significanclty more alkaline earth ions compared to the ion exchange resin, and vice versa. Accordingly, the ion exchange reactions occur mainly in the direction of dissolving sodium ions into water, meaning from left to right in the above reaction formulas.
The ion exchange resin becomes loaded with calcium ions and magnesium ions as described above, and at the same time releases a chemically equivalent amount of sodium ions into the water. The sodium ions remain in the softed water. This means that softening is not a demineralisation process. Instead, the concentration of sodium ions in the water is increased by the chemical equivalent of the removed alkaline earth ions, meaning that the chemical equivalent of the todal dissolved solids remains the same.
With increasing load of the ion exchange resin by calcium and magnesium ions, the concentration gradient between water and ion exchange resin as described above is reduced. Accordingly, the ratio between the ion exchange reactions occuring in one and in the other direction as described above changes. From a certain point onwards, the total balance of the calcium and magnesium ions exchanged will be significantly reduced. At this point, the ion exchange resin is depleted, and needs to be regenerated.
For regeneration, a sodium chlorid brine flows through the resin bed. This results in the the following ion exchange reactions:
2Na+(aq) + 2Cl-(aq) + Ca2+— [resin] ↔ Ca2+(aq) + 2Cl-(aq) + 2Na+— [resin]
2Na+(aq) + 2Cl-(aq) + Mg2+— [resin] ↔ Mg2+(aq) + 2Cl-(aq) + 2Na+— [resin]
The concentration gradient is now reversed, meaning that the ion exchange contains significantly more calcium and magnesium ions and significantly less sodium ions than the regeneration soltuion, and vice versa. Accordingly, the ion exchange reactions now mainly occur in the direction of exchanging calcium and magnesium ions from the ion exchange resins with sodium ions from the regeneration solution. After regeneration, the ion exchange resin is again mainly loaded with sodium ions.
In addition to the alkaline earth ions calcium (Ca2+) and magnesium (Mg2+), other cations are also exchanged by the ion exchange resin, for example the alkaline earth ions barium (Ba2+) and strontium (Sr2+), dissolved iron (Fe2+ or Fe3+), dissolved copper (Cu+ or Cu2+), and ammonia (NH4+). These cations however cannot be fully removed by regeneration with sodium chloride, meaning that the capacity of the ion exchange resin is gradually reduced in case of raw water containing higher concentrations of these ions.
| soft water volume flow |
available from approx. 0.5 up to 350 m³/h for each line condensate softener from approx. 1.5 up to 550 m³/h for each line |
||
| amount of lines | usually 2x100% | ||
| soft water quality | water hardness |
< 0.01 mmol/L, counter-flow regeneration (¹) < 0.02 mmol/L, co-flow regeneration (¹) |
|
| differential pressure at nominal flow | approx. 1.0 ... 1.5 bar | ||
| operating temperature |
usually 5 ... 40 °C condensate softening ≤ 110 °C |
||
| regeneration agent | sodium chloride (NaCl) | ||
| regeneration intervals |
usually 6 ... 24 h condensate softening often ≥ 24 h |
||
| regeneration duration | approx. 60 ... 120 minutes | ||
| chemical consumption |
approx. 200 ... 250 g NaCl for each 1 mol Ca2+, counter-flow regeneration (²) approx. 340 ... 400 g NaCl for each 1 mol Ca2+, co-flow regeneration (²) |
||
| regeneration effluent |
approx. 6 ... 10 L for each 1 mol Ca2+, counter-flow regeneration (²) approx. 10 ... 15 L for each 1 mol Ca2+, co-flow regeneration (²) |
||
| recommended raw water quality | total suspended solids (TSS) |
< 0.5 ... 1 mg/L, counter-flow regeneration < 1 ... 5 mg/L, co-flow regeneration |
|
| iron | < 0.3 mg/L Fe | ||
| manganese | < 0.1 mg/L Mn | ||
| free chlorine | < 0.1 mg/L Cl2 | ||
| oil, grease | < 0.1 mg/L | ||
| ion exchange medium |
• cation exchange resin, strong acid, gel, polystyrenic • cation exchange resin, strong acid, macroporous, polystyrenic |
||
| total service life of the ion exchange resins | usually ≥ 7 ... 10 years | ||
| material options | pressure vessel |
• glass-reinforced plastic (GRP) • carbon steel (e.g. S235JR, P265GH) • stainless steel (e.g. 1.4404, 1.4571) |
|
| brine storage tank | • polyethylene (PE) | ||
| pipelines |
• polyvinyl chloride (PVC) • polypropylene(PP) • stainless steel (e.g. 1.4404, 1.4571) |
||
| valves |
• polyvinyl chloride (PVC) • polypropylene(PP) • brass (e.g. CW610N) • stainless steel (e.g. 1.4408) |
||
| gaskets |
• etyhlene propylene diene monomer rubber (EPDM) • polytetrafluoroethylene (PTFE) • NBR composite |
||
| control options | regeneration, line change-over |
• fully automated, via micro-processor control unit • fully automated, via PLC • manual operation (usually not recommended) |
|
| process monitoring |
• volume counter (standard) • differential pressure (option) • level brine storage tank (option) • residual water hardness (option) |
||
| ¹ Water hardness: 0.01 mmol/L ≈ 0.05 °dH ≈ 1 mg/L CaCO3, see →unit conversions. | |||
| ² Molar and equivalent quantities: 1 mol Ca2+ ≈ 5.6 °dH m³ ≈ 0.1 kg CaCO3, see →unit conversions. Example: With co-flow regeneration, a raw water hardness of 200 mg/L CaCO3 ≈ 2 mmol/L, and a soft water volume flow of 10 m³/h, the mean salt consumption would be 2 mol/m³ ⋅ 10 m³/h ⋅ 340 ... 400 g NaCl = 6,8 ... 8,0 kg/h NaCl, and the mean effluent discharge would be 2 mol/m³ ⋅ 10 m³/h ⋅ 10 ... 15 L = 200 ... 300 L/h. | |||
2018-05-05 • water treatment made in Germany • Company Information • Privacy